ATP is a universal energy storing molecule in biological systems. How does life generate ATP from ADP? Similarly, how does life consume it on an industrial scale in membrane bound systems such as mitochondria and chloroplasts? ATP synthase (or ATPase depending on which catalytic direction you look at it) enzyme is a hugely interesting molecular structure. Yes it most certainly is a bi-directional turbine. It is one of the few rotatory molecular structures life has evolved such as the flagella rotator disc in bacteria.
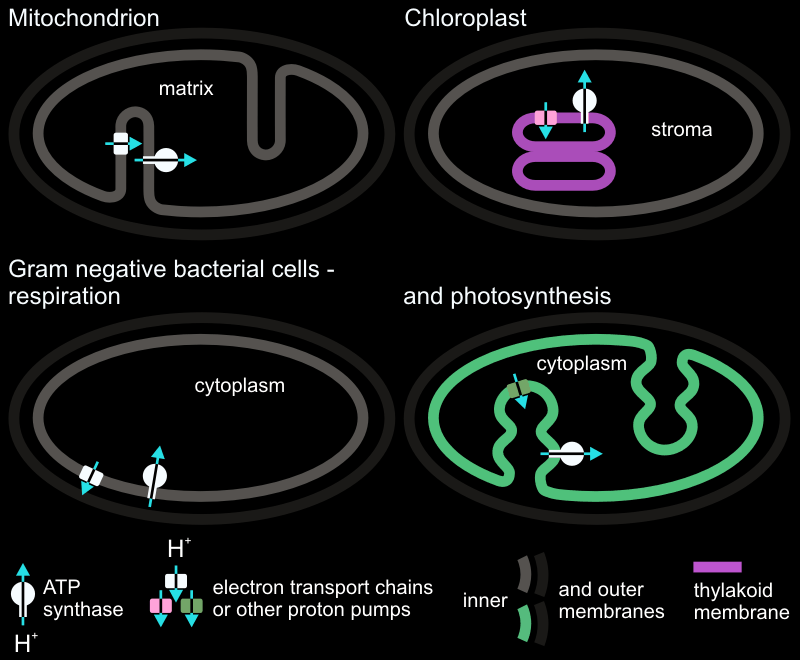
The turbine analogy makes sense when we investigate the F0 electric motor portion of the assembly. The rotor in F0 has 12 identical protein chains that function just like the fan blades in a turbine. Human designed turbines use gas or water as drivers. Shaped under evolutionary selective pressures the ATP synthase/ATPase uses hydrogen ions. The chemiosmotically maintained gradient of hydrogen ions across organelle membranes by the electron transport chain drives the “blades” of the F0 rotor.
The ion pump that feeds hydrogen ions into F0-rotor is a single protein chain. The pump protein has an arginine amino acid that enables flow of a hydrogen ions to aspartate residues on the F0-rotor. Aspartate amino acids are negatively charged. The F0-rotor is insulated by membrane lipids. Just like steam or water acting on turbine blades, the F0 rotor turns when the aspartic acid residues have a hydrogen attached. This neutralizes their charge. Hydrogen ions take a path not yet elucidated through the F0 motor and turn the rotor. Proton-motive force drives the ATP generation.
There are at least 3 types of ATP synthase/ATPases evolved to operate on diverse membrane-bound compartments such as vesicles, vacuoles, photosynthetic stroma and plasma membranes.
The A-ATPases are found in Archea. The V-ATPases are found in vesicular structures and vacuoles. The F-ATPases as shown in this video are among the most well characterized group found in eukaryotic mitochondria and chloroplasts. Prokaryotic P-type ATPase are a different enzyme group however. P-type ATPase are found on plasma membranes and do not generate ATP. Discovered in 1957, P-type prokaryotic ATPases function purely as ion pumps by consuming ATP.
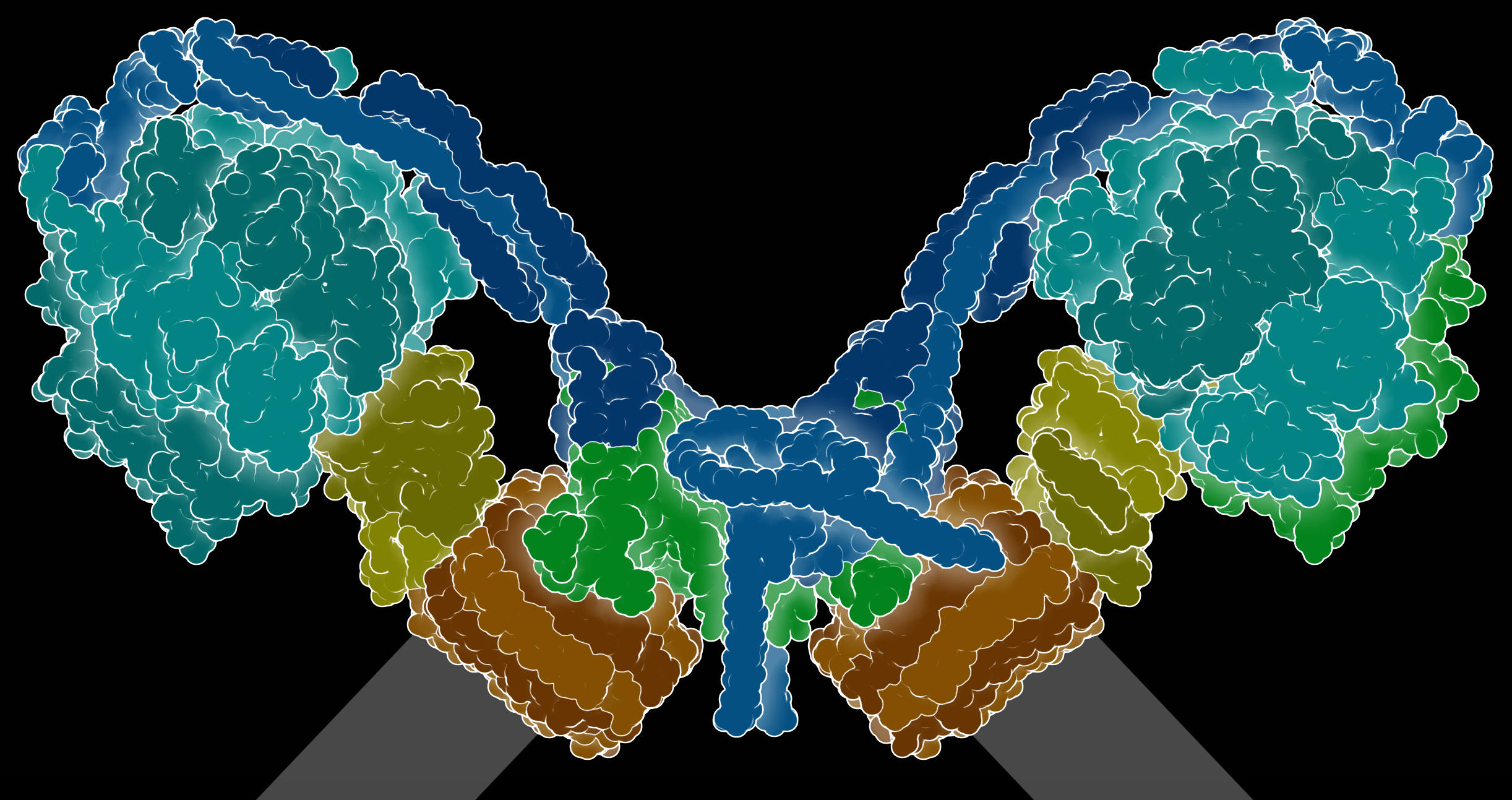
Most of the time ATP synthases are depicted in a simplified manner as a single unit. However in reality they are found in dimer form. Cryo-electron microscopy studies have revealed how the multi-subunit ATPase complexes are assembled in yeast and humans. ATPase enzyme structure has evolved striking differences in various organisms. One parasitic unicellular organism (Euglena gracilis) that causes sleeping sickness for instance, appears to have evolved an unusual ATP synthase in shape and size. The enzyme is larger than other well studied organisms such as the Human and the yeast and 13 of its 29 subunits are specific to the Euglenaceae family. Some of these distinctive parts are held together by cardiolipins, which appear to create a shape in the inner mitochondrial membrane increasing ATP synthase efficiency. The more we look the more fascinating the subject matter becomes.
A landmark publication by Mitchell in 1961 explained oxidative and photo-phosphorylation mechanism. This changed the dynamics of a heated debate known as “OxPhos Wars”. There appeared to be a connection between the respiratory and photosynthetic electron transport chains. Indeed these two seemingly divergent processes were showing a resemblance in ATP generation. The production of ATP was not dependent on synthesis of an energy-rich intermediate compound. Biochemists of the time (since mid 1950s) were wrongly seeking for a straightforward chemical reaction similar to that of the glycolysis where reactants and products were highly balanced. Mitchell laid the foundation that ATP generation resulted from an organized chain of respiratory complexes and ultimately catalyzed by ATP synthase.
A virtual tour of a cell
Mitochondria: the cell’s powerhouse
Electron Transport Chain


0 Comments
You can be the first one to leave a comment.